Cross-Linking FAQ: How Long Does it Take to Recover From iLink®?
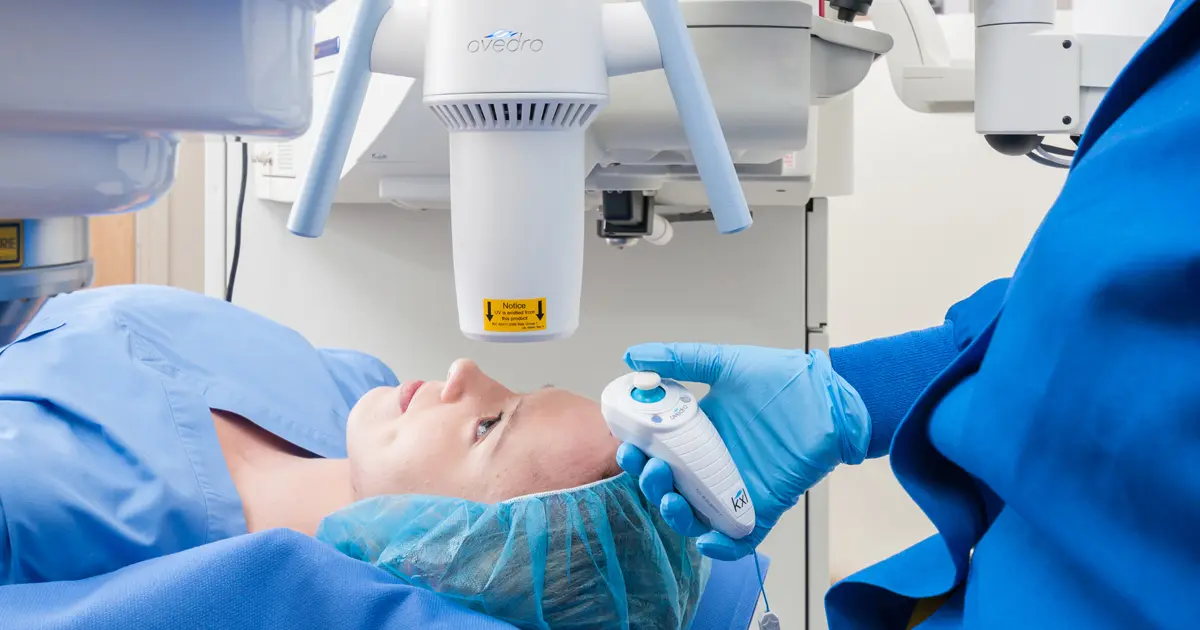
If you or someone you know has keratoconus, it is likely that you have heard of iLink® FDA-approved corneal cross-linking. For those who aren’t familiar with this treatment option, iLink® is the only FDA-approved cross-linking procedure for the treatment of progressive keratoconus. iLink® is a minimally invasive outpatient procedure to slow or halt the progression of the condition.
As with any medical procedure, you probably have a number of questions circling through your head, such as, “What will the recovery be like?” You may be wondering how much time you will need to take off from work or school, and if there is anything you may not be able to do or if your activities will be limited during your recovery. When it comes to iLink®, we want to help answer as many of your questions as possible. Continue reading to learn more about how long it takes to recover from FDA-approved cross-linking and what that process may look like for you.
How long does it take to recover from cross-linking and what does recovery look like?
After undergoing iLink® FDA-approved cross-linking, you will want to give yourself some time to rest and recover before jumping back into your daily activities. It’s likely that you will want to take two to three days off from work or school to recover, but everyone’s experience is different. If you or someone you know is trying to determine when to undergo the procedure, summer can be an ideal time to receive cross-inking to allow for additional recovery time without having to miss school or other commitments. You likely won’t want to rush to return to work or school if you’re experiencing any discomfort, which often occurs during the immediate recovery, but not usually during the treatment itself. Any discomfort is typically experienced after the numbing drops from the procedure wear off and can be managed with Tylenol®, artificial tears and ice packs. If the pain is severe, oral narcotic medications may be used. You should contact your physician immediately if you experience severe pain in the eye or any sudden decrease in vision.
Following the procedure, you will likely be fitted with a temporary bandage contact lens. If your bandage lens from the day of treatment falls out or becomes dislodged, do not replace it and make sure to contact your physician immediately. People with keratoconus should always avoid rubbing their eyes, but especially during the first five days after the procedure. During this time, you might notice some sensitivity to light, which can be reduced by wearing sunglasses. You may also notice a temporary “foreign body”-type sensation. This is normal and should alleviate as your recovery progresses. If you wear contact lenses, you may not be allowed to use them for up to about one month after the procedure in the treated eye, but, but your doctor will give you specific instructions on this as well.

“Typically, the patient will have a soft contact applied immediately after the procedure as a bandage. This will stay in place until the epithelium heals, which may be between one day and one week. The eye will be scratchy and irritated for that time until the epithelium heals, but the contact reduces this significantly. The patient does not have many restrictions after the procedure. The first few days are just limited by comfort and vision. Being that the patient is not wearing their pre-op contacts for at least one week, they may have trouble [seeing] due to their refractive error, unless they can see well in glasses. From an activity standpoint, they really have no restrictions.”
Dr. Kenneth Beckman, Comprehensive Eyecare of Central Ohio
Recovery from iLink® FDA-approved cross-linking will be different for everyone. Make sure to talk to your doctor about what recovery might look like for you, and how long you should expect to forgo certain activities. If you’re interested in learning more about iLink®, visit our website or check out any of our recent blogs where we’re answering your top cross-linking questions. Also, don’t forget to follow us on Facebook, Twitter, and Instagram!