Should You Seek a Second Opinion Before Receiving Cross-Linking?

With so much information available at our fingertips – from physicians, family and friends, and the internet – finding a treatment path that is right for you may seem overwhelming. For those living with progressive keratoconus, solutions and treatments can range from an updated lens prescription to corneal cross-linking, or a corneal transplant for more advanced cases of the condition. If it is determined that iLink® FDA-approved cross-linking is the right treatment for you, the procedure offers the potential to slow or halt progression of the condition to preserve vision. Slowing progression is an important step to enable people to remain in contact lenses, and hopefully avoid corneal transplantation.
However, people may be surprised to learn that there are various cross-linking treatments being performed in the U.S. – some FDA-approved and some not. In fact, some members of the keratoconus community are unaware that the cross-linking option they have been offered has not been approved by the FDA. Because of this, people need to advocate for their own eye health and ask whether they are receiving iLink, the only FDA-approved cross-linking treatment for progressive keratoconus.
This is where getting a second opinion may come into play. If you’ve never asked for a second opinion before, the decision can feel overwhelming, so we’re here to help. Continue reading to determine if you should receive a second opinion on your recommended keratoconus treatment options, and to learn some questions that you should ask around the cross-linking procedure you are being offered to ensure you are receiving the FDA-approved treatment.
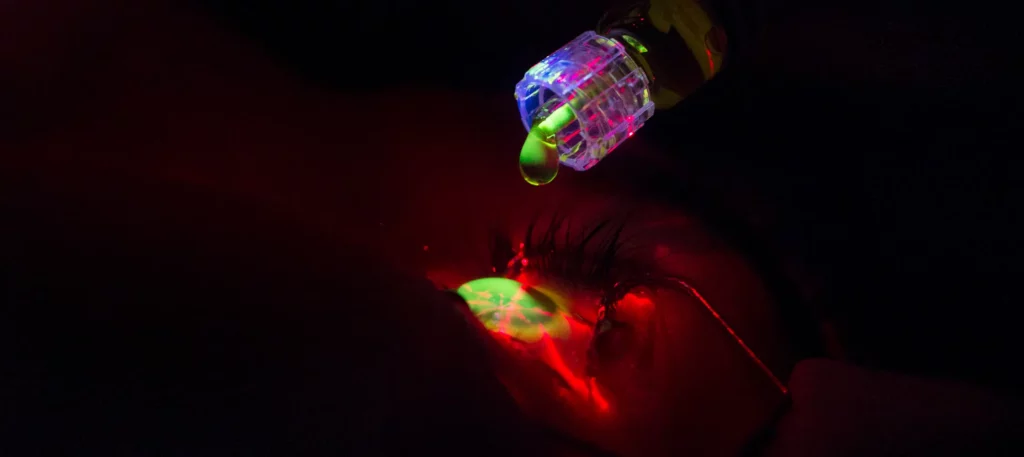
Why It’s Important to Have All the Information
When it comes to treating progressive keratoconus, did you know that not all cross-linking procedures are the same? It may seem shocking, but even though your doctor may offer cross-linking, that does not necessarily mean they are performing iLink, the only FDA-approved cross-linking procedure.
iLink FDA-approved cross-linking is the ONLY cross-linking treatment proven safe and effective in slowing or halting the progression of keratoconus to help preserve vision. The iLink epi-off procedure is also the only cross-linking treatment approved by the FDA (since April 2016) and eligible for insurance coverage. This minimally invasive, outpatient procedure combines the use of prescription eye drops, Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution), and ultraviolet A (UVA) light from the KXL® system to treat progressive keratoconus. It works by creating new collagen cross-links and leads to the stiffening of the cornea.
There are other cross-linking procedures being offered that use eye drops and medical devices that are NOT FDA-approved, so it’s important to understand the distinction. If a cross-linking procedure is unapproved or investigational, that means that the procedure and its manufacturing process have not been thoroughly evaluated by the FDA for safety and effectiveness and is not eligible for insurance reimbursement. While manufacturers of FDA-approved drugs and devices are required to report adverse events to the FDA, there is no equivalent reporting required for unapproved products, which may present a risk to public safety.
A doctor may offer corneal cross-linking, but that does not necessarily mean that it is iLink FDA-approved cross-linking. Make sure you know the difference and what questions to ask your doctor!
Not sure if your doctor is offering iLink? Check out our physician locator to make sure your doctor is listed! This list includes U.S. physicians performing iLink FDA-approved cross-linking.
When to Consider a Second Medical Opinion
Seeking a second opinion doesn’t mean you don’t like your doctor or even disagree with him or her. It simply means that you are taking control of your health and trying to learn as much information as you can to make an informed and educated decision on your treatment pathway.
When it comes to treating your keratoconus, it is important to ensure you are receiving the FDA-approved cross-linking procedure. You may want to consider seeking a second opinion about the procedure offered to you if:
- Your doctor recommends a cross-linking procedure that has not been FDA-approved.
- Only iLink cross-linking with the Photrexa drug formulations and the KXL System are approved by the FDA.
- Don’t risk your vision on a product or procedure that does not have proven safety and efficacy. If your keratoconus is not effectively treated and continues to progress, you may lose vision that cannot be recovered. Ask your doctor if iLink may be right for you.
- Your doctor asks you to pay cash and submit to insurance on your own OR your doctor tells you that cross-linking is not covered by insurance.
- iLink FDA-approved cross-linking is widely covered by insurance and is the ONLY cross-linking procedure eligible for reimbursement in the U.S.
- Greater than 95% of the commercially insured population in all 50 states has coverage for the iLink procedure.
- If your doctor is using iLink FDA-approved cross-linking and you meet medically necessary criteria, they will be able to bill your insurance directly, in most cases.
- Your doctor suggests you pay for treatment as part of their own trial.
- Clinical trials leading to FDA-approval do not typically charge patients out of pocket for treatment or post-operative care.
- A clinical trial for an investigational drug conducted with only the approval of an Institutional Review Board (IRB), and not conducted under an IND that has been made by the FDA, is unlawful and illegal, except for limited exceptions.
- You should ask for proof that the FDA has granted the practice permission to charge for the investigational drug.
Not sure if your doctor is offering iLink? Check out our physician locator to make sure your doctor is listed! This list includes U.S. physicians performing iLink FDA-approved cross-linking.
Questions You Should Be Asking About Cross-Linking
Before seeking a second opinion, or even during an appointment with a new physician, make sure you are prepared! Spend some time brainstorming a list of questions to ask about keratoconus and available treatment options, including iLink FDA-approved cross-linking for progressive keratoconus. To make sure you’re receiving iLink, consider the following questions:
- Is my doctor performing the cross-linking treatment that is FDA-approved and covered by insurance?
- What are the key differences between iLink and non-FDA-approved procedures?
- Does insurance coverage matter to me?
- What’s more important than my sight?
Having the answers to these questions may help make the decision about whether or not to receive the cross-linking procedure that you are being offered.
Be Proactive!
Planning and scheduling your cross-linking procedure shouldn’t be a scary process. Don’t be afraid to take your health into your own hands! If something seems off about the treatment your physician is offering and they are not giving you direct answers, or if you are being told that you need to pay out of pocket for your cross-linking procedure, you may want to seek a second opinion.
If you want a second opinion, but are unsure where to start, begin by looking for a new doctor. Use our physician locator to find a physician near you that is performing iLink FDA-approved cross-linking. You can also visit our website to learn more about the iLink procedure and available insurance coverage. In addition, don’t forget to follow us on Facebook, Twitter, and Instagram for more information!