Curious About Insurance Coverage for Cross-Linking? Here’s What to Know!
We all know that health insurance coverage can be a pain to navigate, regardless of the situation! But it shouldn’t be when it comes to covering your cross-linking procedure to slow or halt the progression of your keratoconus. Even though more than 95% of the commercially insured population have access to iLink® cross-linking, many people are still unaware that the FDA-approved procedure is actually widely covered by insurance.
To help clear up any confusion, we’re highlighting everything you need to know about insurance coverage for iLink® FDA-approved cross-linking, including some frequently asked questions and if you should seek a second opinion on the treatment you’re being offered.
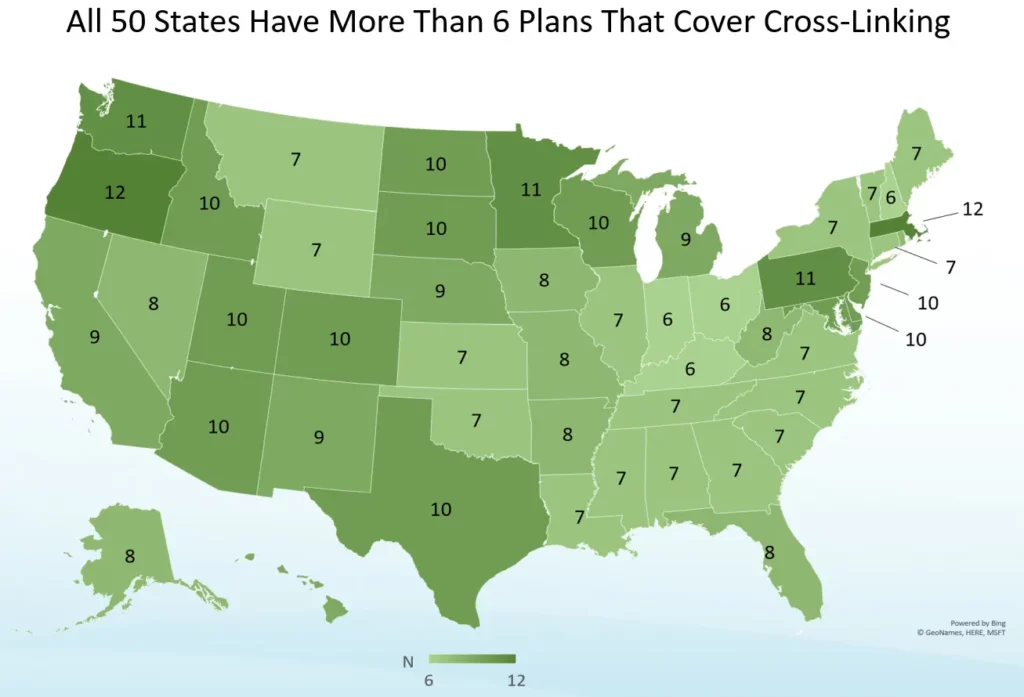
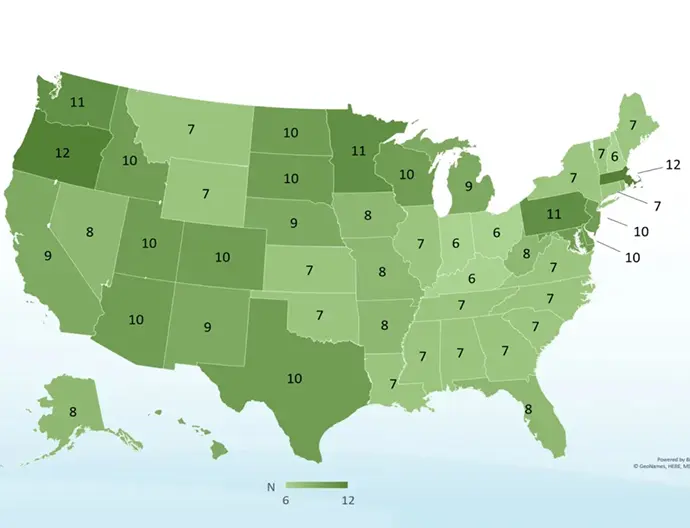
Insurance Coverage for iLink® FDA-Approved Cross-Linking
The goal of iLink® FDA-approved cross-linking is to stiffen the cornea to slow or prevent further progression of keratoconus and preserve your vision. Because of this, insurance coverage for FDA-approved cross-linking with Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution), and ultraviolet A (UVA) light from the KXL® system is now widely available, and there are positive coverage policies from the largest national commercial payers in the U.S., including United Healthcare, Anthem Blue Cross Blue Shield, Aetna, Cigna, Humana, and HealthNet. In fact, all 50 states have more than six insurance plans that cover the FDA-approved procedure.
For most insurance policies, corneal cross-linking is medically necessary for someone who has progressive keratoconus. It is also considered medically necessary for those with corneal ectasia post-refractive surgery with documented worsening best spectacle-corrected visual acuity and irregular astigmatism.
Commonly Asked Questions
We know you may still have some questions about insurance coverage, as the process can be tricky, so we want to help! Below are some answers to your commonly asked questions about insurance coverage for FDA-approved cross-linking.
Does insurance cover FDA-approved cross-linking?
Insurance coverage for iLink® FDA-approved cross-linking is widely available. Greater than 95% of the commercially insured population has access to this potentially sight-preserving treatment.
Does insurance cover non-FDA-approved products?
Insurance does not typically cover products and procedures that have not been approved by the FDA. As an example, the Blue Cross Blue Shield Association’s Technology Evaluation Center (Premera BCBS Technology Review) lists as part of their new technology evaluation criteria: “1. The technology must have final approval from the appropriate governmental regulatory bodies.”
Does insurance cover epi-on procedures?
The only FDA-approved products for cross-linking are from Glaukos, which are performed epi-off. Many insurance policies detail the fact that the epi-off procedure is the only FDA-approved treatment for progressive keratoconus that is covered, while epi-on is not.
Which insurance companies cover corneal cross-linking for the treatment of progressive keratoconus?
The list of companies that are known to have policies that cover cross-linking to treat progressive keratoconus includes these large national insurers – United Healthcare, Anthem Blue Cross Blue Shield, Aetna, Cigna, Humana, and HealthNet.
How will I know if my insurance covers corneal cross-linking?
Contact your insurance provider prior to treatment to see if they currently provide coverage for iLink® FDA-approved corneal cross-linking. Glaukos works directly with doctors’ offices and insurance companies to help facilitate the insurance reimbursement process. Prior to undergoing the procedure, confirm with your doctor that they are performing the iLink® FDA-approved cross-linking procedure. You can find a list of doctors who are familiar with the procedure by using our locator tool.
If I receive cross-linking as part of a clinical study, will it be covered by my insurance?
No, insurance companies typically require FDA-approval to determine coverage. Additionally, clinical studies evaluating investigational procedures are typically offered to the patient at no cost.
Should You Get a Second Opinion?
Since iLink® is widely covered by insurance, your physician should not require you to pay for the procedure out of pocket. If your doctor indicates that you will need to pay out of pocket, you should question it and consider a second opinion. It is likely that they do not offer iLink® FDA-approved cross-linking. Instead, they may offer a procedure that is unapproved or investigational.
Seeking a second opinion doesn’t necessarily mean that your doctor is entirely wrong. It does mean, however, that you are taking control of your health and trying to learn as much information as you can to make an informed and educated decision. Here are some guidelines to help you determine if you should seek a second medical opinion:
- Your doctor asks you to pay cash and submit to insurance on your own.
- Your doctor suggests that you pay for treatment as part of his or her own trial (clinical trials for FDA approval are typically free to patients).
- Your doctor tells you that his or her cross-linking procedure is not covered by insurance.
Not sure if your doctor is offering iLink®? Check our physician locator to make sure your doctor is listed, or to find another physician in your area that is performing the FDA-approved procedure!
Don’t Be Discouraged!
Don’t let someone tell you that FDA-approved cross-linking is not covered by insurance! Everyone’s insurance situation is unique and if your insurance hasn’t posted a coverage policy, it doesn’t necessarily mean they do not cover the procedure. It’s common that claims may be rejected due to incomplete information when submitted, such as failure to submit documentation of the progression of keratoconus.
Ask your doctor to consult with your insurance company to determine coverage or contact your insurance company directly to speak to a benefits representative. You can also ask your doctor to file an appeal, and depending on your situation, that appeal may be done before or after treatment. You may also discuss financing options with your doctor.
As always, don’t forget to follow us on Facebook, Twitter, and Instagram!