John’s “Crazy” Keratoconus Journey

Ever since he was a young boy, John, now 26 years old, has always loved making people smile. As he got older, it brought him such joy to make others happy that he decided to make a career out of it. John, who has progressive keratoconus and Down syndrome, teamed up with his father, Mark, to establish their own business. Together they launched John’s Crazy Socks, a social enterprise inspired by John’s love of colorful and fun socks with a mission to make people happy, while also raising money for important causes. Although John was optimistic about his future, he was worried that his keratoconus would potentially impact the career he is so passionate about. Keep reading to learn more about John’s inspiring journey to treating his keratoconus and running a successful and charitable business, all while making a difference in people’s lives.
Creating His Own Path
John’s focus has always been to make others happy, but when he began experiencing significant vision issues in junior high school, he had to focus on his own life. He was having trouble seeing the boards at school, and his parents noticed that John was squinting frequently. After being seen by an eye doctor, John was simply fitted with glasses and sent on his way. Unfortunately, his eye problems persisted and John noticed that his prescription was no longer working. After scheduling another eye exam, John was diagnosed with progressive keratoconus.
Even though he had never heard of keratoconus, John and Mark learned that it is not uncommon for people who are living with Down syndrome to experience eye issues. In fact, research shows that 5 to 15% of people with Down syndrome are affected by keratoconus, even though it is considered a rare disease. Although it was comforting to know what was going on, the duo was disappointed to learn that there were not many treatment options available to them at the time.

Despite being in the middle of navigating his keratoconus journey, John was also concentrated on his future and career. In September 2016, John entered his last year of school, but he struggled to find work that he could pursue after leaving school. Instead of letting it get him down, he decided to go into business with his father. By December of that year, John and Mark opened John’s Crazy Socks, a specialty sock company that raises money for important causes such as Down syndrome, breast cancer, animal rescue, and more. In addition, 5% of all sales are donated to the Special Olympics. John’s Crazy Socks also serves as an outlet for John to express his creativity. When the company first launched, they offered 37 different sock styles and patterns. Today, as the world’s largest sock store, John’s Crazy Socks carries more than 4,000 varieties of socks and has been featured on national news stations, including ABC, BBC, CBS, PBS, and more.
Getting Proper Treatment
While the business was booming, John’s keratoconus continued to progress. In search of a keratoconus expert to advise them on appropriate treatment options, John and Mark met Dr. Ann Ostrovsky. Dr. Ostrovsky is an Assistant Clinical Professor of Ophthalmology at New York University Medical Center and Director of the Keratoconus Program at NYU Langone Eye Center, where she specializes in collaboration with the NYU Down Syndrome Program and pediatric ophthalmologists. Upon examining John’s keratoconus, Dr. Ostrovksy recommended he receive iLink® FDA-approved cross-linking to slow or halt the progression of the condition in his right eye. Unfortunately, the progression of keratoconus in John’s left eye was too advanced for cross-linking. For the time being, Dr. Ostrovksy continues to monitor John’s left eye and he is using a contact lens to help his vision.
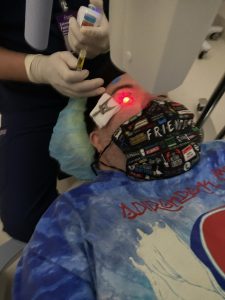
Before the surgery, Dr. Ostrovsky walked John and Mark through every step of the cross-linking procedure so that John would know exactly what to expect. Then, on the day of the procedure, Dr. Ostrovsky played John’s favorite music to make him even more comfortable. John underwent iLink FDA-approved cross-linking in his right eye on August 3, 2021. He experienced minor pain afterward but felt it was manageable overall. Following the procedure, John was fitted for a scleral lens to help correct his vision. It was then that Mark began to notice improvements[1] in John’s vision, such as less squinting, and John seemed more comfortable.
There’s Nothing Holding Him Back
Since receiving iLink FDA-approved cross-linking and being fitted with a scleral lens, John is now free to focus on his career and future without having to worry about his keratoconus progressing. He has goals to grow his business this year, travel, speak at in-person events, and maybe even attend the Special Olympics. He’s also thinking about designing a special sock to help spread awareness for keratoconus. No matter what the year brings, John’s happy he received iLink and knows that he can handle anything that comes his way next.
[1] Kreps, E. O., Pesudovs, K., Claerhout, I., & Koppen, C. (2021). Mini-Scleral Lenses Improve Vision-Related Quality of Life in Keratoconus. Cornea, 40(7), 859–864.
Find a Cornea Cross-Linking Specialist Near You:
The results described on this site are based on data collected regarding short- and intermediate-term efficacy of treatment. Individual results are not guaranteed and may vary.